The role of the cold-shock protein YB-1 in hyperproliferation of leukemic T cell blasts
Projektleiter:
Projektbearbeiter:
S. Gieseler,
S. Meltendorf,
M. Pierau,
M.C. Brunner-Weinzierl
Finanzierung:
Stiftungen - Sonstige;
Summary of the project:
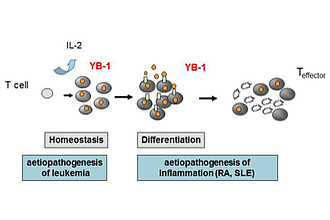
Analysis of the function of YB-1 in peripheral and malignant CD4+ T cells, and its contribution to the pathogenesis of T cell leukemia and chronic inflammation (Fig. 1).
Introduction:
The cold-shock protein YB-1 is an oncogenic transcription/translation factor highly expressed in tumor cells of breast, ovarian, and lung cancer. It correlates with their increased cell survival, proliferation, and migration (1, 2). Its enhanced expression of mRNA and its localization within the nucleus has been shown to correlate with poor prognosis for breast cancer patients (3). Although YB-1 plays a central role in tumor etiopathogenesis its role in T cell leukemia and T cell responses is not understood, yet.
Results and discussion:
We identified that YB-1 is unambiguously expressed in primary and malignant human T cells and -lines of patients suffering from T-ALL, a prototype of a non-solid cancer. It´s location in the nucleus correlated well with proliferation in malignant T cell lines of T-ALL patients. In primary T cells, enhanced expression appeared already in G1 phase of the cell cycle and was enhanced by stimulation, especially co-stimulation by CD28 via activation of RSK leading to YB-1 S102 phosphorylation, thus identifying the MAPK signaling pathway as a prerequisite for YB-1 translocation into the nucleus. In addition, shRNA/siRNA-mediated knock-down of YB-1 or inactivated RSK resulted in abrogated proliferation of T cells that could not be rescued by IL‑2. In bone marrow of first diagnosed T-ALL patients, nuclear YB-1 content was strictly reduced compared to controls, indicating a silencing process for bone marrow resident leukemic cells which might provide a novel clue for both evaluation of disease activity and a potential target for individualized therapy. All together YB-1 is tightly controlled in T cells by co-stimulation and is centrally involved in cell cycle progression of T cells.
Perspectives:
The future directions of the project are the analysis of YB-1 in T cell differentiation and its contribution to chronic inflammation and systemic rheumatic diseases.
Analysis of the function of YB-1 in peripheral and malignant CD4+ T cells, and its contribution to the pathogenesis of T cell leukemia and chronic inflammation (Fig. 1).
Introduction:
The cold-shock protein YB-1 is an oncogenic transcription/translation factor highly expressed in tumor cells of breast, ovarian, and lung cancer. It correlates with their increased cell survival, proliferation, and migration (1, 2). Its enhanced expression of mRNA and its localization within the nucleus has been shown to correlate with poor prognosis for breast cancer patients (3). Although YB-1 plays a central role in tumor etiopathogenesis its role in T cell leukemia and T cell responses is not understood, yet.
Results and discussion:
We identified that YB-1 is unambiguously expressed in primary and malignant human T cells and -lines of patients suffering from T-ALL, a prototype of a non-solid cancer. It´s location in the nucleus correlated well with proliferation in malignant T cell lines of T-ALL patients. In primary T cells, enhanced expression appeared already in G1 phase of the cell cycle and was enhanced by stimulation, especially co-stimulation by CD28 via activation of RSK leading to YB-1 S102 phosphorylation, thus identifying the MAPK signaling pathway as a prerequisite for YB-1 translocation into the nucleus. In addition, shRNA/siRNA-mediated knock-down of YB-1 or inactivated RSK resulted in abrogated proliferation of T cells that could not be rescued by IL‑2. In bone marrow of first diagnosed T-ALL patients, nuclear YB-1 content was strictly reduced compared to controls, indicating a silencing process for bone marrow resident leukemic cells which might provide a novel clue for both evaluation of disease activity and a potential target for individualized therapy. All together YB-1 is tightly controlled in T cells by co-stimulation and is centrally involved in cell cycle progression of T cells.
Perspectives:
The future directions of the project are the analysis of YB-1 in T cell differentiation and its contribution to chronic inflammation and systemic rheumatic diseases.
Schlagworte
leukemic T cell
Kontakt

Prof. Dr. habil. Monika Christine Brunner-Weinzierl
Otto-von-Guericke-Universität Magdeburg
Experimentelle Pädiatrie und Neonatologie
Leipziger Str. 44
39120
Magdeburg
Tel.:+49 391 6724003
weitere Projekte
Die Daten werden geladen ...

